Carbon is the basis of this new material
Of all the elements on Mendeleev’s periodic table, it is carbon that forms the most different molecules. This property is due to its ability to create 4 simultaneous chemical bonds with other atoms, but also with itself. This quadrilateral allows it to form many structures.
Carbon is an essential compound for life. It is such a part of the composition of the majority of organic molecules that we sometimes talk about carbon chemistry to talk about organic chemistry.
>> Read also: “Why does life depend on carbon?”
In its crystalline form, pure carbon exists in two allotropic forms: diamond and graphite. Diamonds consist of transparent tetrahedral structures. They are formed under conditions of extremely high temperatures and pressures. In these tetrahedral structures, the bonds between carbon atoms are extremely strong, which explains the hardness of diamonds, which, on the Vickers scale, ranges between 56 and 62 gigapascals (GPa). Diamond is also an insulator that does not allow electric current to pass through.
On the other hand, under normal pressure conditions, carbon turns into graphite, made up of a stack of flat hexagonal crystal structures called graphene sheets. Graphite is a completely friable material, due to which the different plates slide on themselves due to the low cohesive strength. Unlike diamond, graphite is a conductor of electric current.
Carbon also exists in an amorphous and unstructured form, which is synthesized in the laboratory by evaporation or by evaporation of electron beams. Some, such as the fullerene molecule used in the production of this new, highly resistant glass, are produced in nature during wildfires or in diesel engine emissions.
The elemental carbon is oxidized at a high temperature to form carbon monoxide and carbon dioxide.
>> Read also: “New unbreakable glass inspired by mother-of-pearl!”
Diamond hardened glass
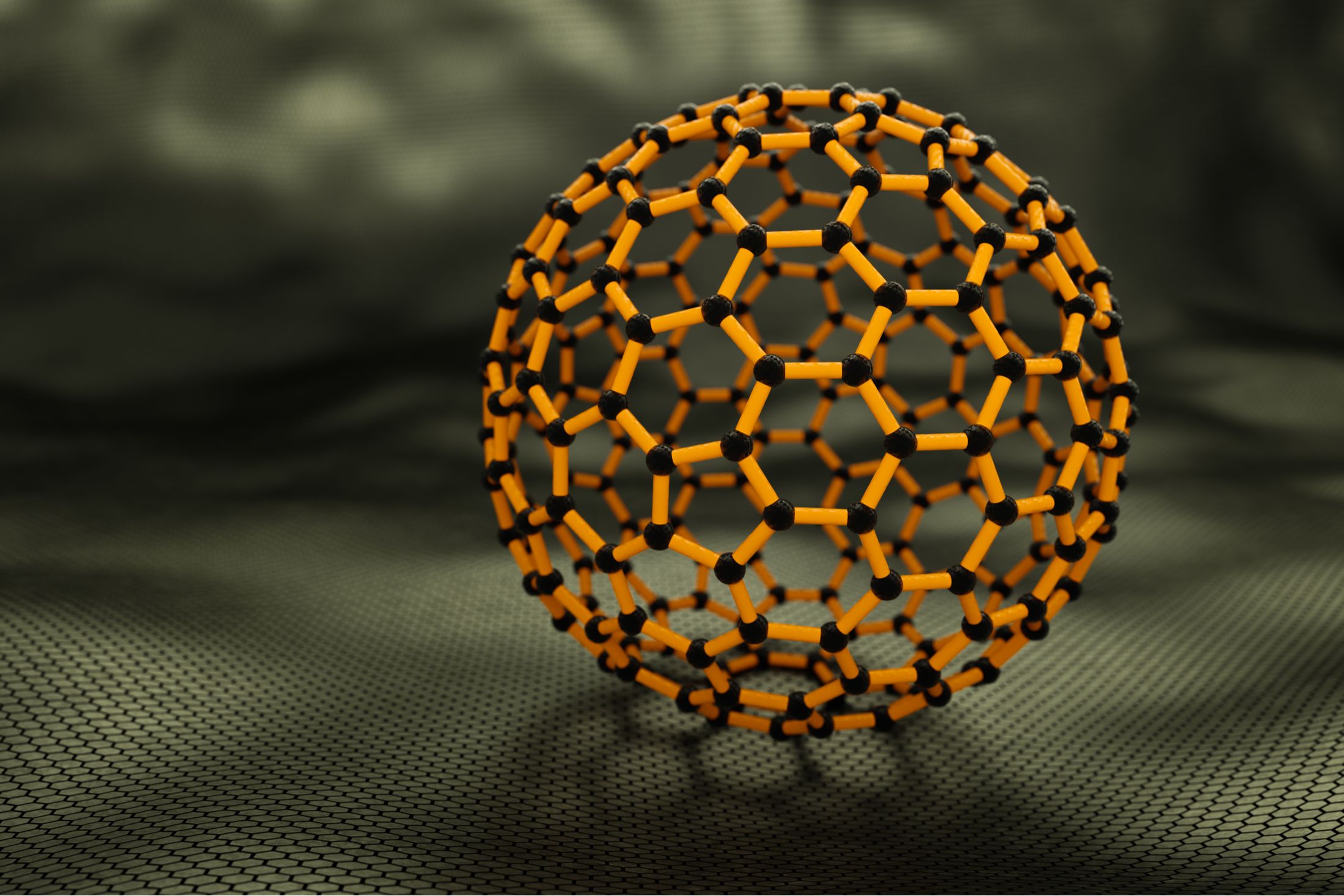
The fullerene family It is a family of compounds containing at least 60 carbon atoms. They take the form of hollow spheres in which the carbon atoms are arranged in the form of a semi-regular polyhedron, in the form of alternating five- and seven-pointed carbon rings.
He discovered the first fullerene molecule, called a ‘buckyball’, and is easily comparable to a type of cage less than a nanometer in diameter. By its composition, it is a very solid molecule that is already finding applications in the pharmaceutical field, in biology or in microelectronics.
To design its highly conductive and highly resistant glass, the researchers tested several configurations of carbon atoms. They chose fullerenes, which consist of 60 carbon molecules in the shape of a hollow sphere.
In order to make such a strong carbon glass, the research team from Jilin University in China introduced particles of Bucky Bowl At high temperatures causing the breakdown of its spherical structure and disturbance within the molecular organization. Then they get a partially amorphous compound. Unlike previous tests that required very high temperatures, the study scientists were able to get results at lower temperatures.
>> Read also: ‘A new carbon material harder than diamond’.
Then the whole was crushed at very high pressure to obtain the desired crystal glass. To create a high pressure, corresponding to about 320,000 times atmospheric pressure, the researchers used a multi-anvil piston. This device is generally used to subject samples of igneous rocks to pressures such as those prevailing at a depth of 800 km, in the upper part of our beautiful blue planet’s mantle.
Then the team of researchers used crystallography techniques such as X-ray diffraction or electron diffraction in order to confirm the properties of this new glass.
This new glass material, a kind of hybrid between amorphous carbon from graphite and crystalline carbon from diamond, is therefore much stiffer than the latter because it is able to leave an imprint on diamond. Its development opens the door to many applications in a variety of fields such as electronics or places where highly resistant glass is required.

“Subtly charming problem solver. Extreme tv enthusiast. Web scholar. Evil beer expert. Music nerd. Food junkie.”