Rapid imaging has seen amazing developments in recent years, particularly with the advent of digital sensors and the miniaturization of electronic circuits. Today there are ultra-fast cameras capable of detecting phenomena of extreme ephemera. We used a camera of this type to examine the details of the mechanisms in action when unscrewing a champagne bottle.
During the second fermentation (called prize de mousse), champagne wine produces carbon dioxide (CO .).2) – equivalent to approximately 5 liters for one 75 cl bottle – which remains trapped under pressure in the closed bottle. The pressure in a bottle that is still corked varies greatly with its temperature. Thus, at 20 ° C, the pressure reaches almost 8 bar, which is 8 times the atmospheric pressure, that is, the pressure that prevails 70 meters below sea level! The video sequence in the image above shows the phenomena that occur following a cork emerging from a bottle under an initial pressure of 8 bar.
When the cork explodes, the volume of carbon dioxide under pressure in the neck of the bottle suddenly expands. Then its pressure goes from 8 bar to an ambient pressure of 1 bar. This is accompanied by a decrease in its temperature: physicists talk about adiabatic expansion. However, depending on its temperature and pressure, a pure substance is likely to exist in three phases: gaseous, liquid and solid. Thus, at a pressure of 1 bar, water becomes liquid at 20 ° C, turns into ice below 0 ° C and boils to steam at 100 ° C. But what about CO2 ? At a pressure of 1 bar, carbon dioxide is CO2 It remains in a gaseous state above a temperature of -78.5 ° C; Below this critical temperature, it exists in its solid form: dry ice.
In unblocked dry ice and shock wave
For this bottle at an initial pressure of 8 bar, the temperature of the carbon dioxide which expands suddenly drops to approximately -90°C. carbon monoxide fumes2 then turns into Small dry ice crystalsCapable of scattering ambient light. The plume’s azure color is a sign of the very small size of these crystals. In fact, particles or particles that are smaller than the wavelengths of the ambient light spectrum (centered around 0.6 micrometers) scatter more effectively the small wavelengths of the spectrum (blue, in particular) than the longer wavelengths (such as red): this is called Rayleigh scattering . It’s the same phenomenon that explains why the sky appears to us blue: the molecules that make up our planet’s atmosphere are much smaller than the wavelengths of sunlight, so the blue color is scattered more efficiently than other colors of the spectrum.
Read more: champagne! The science behind the fizz
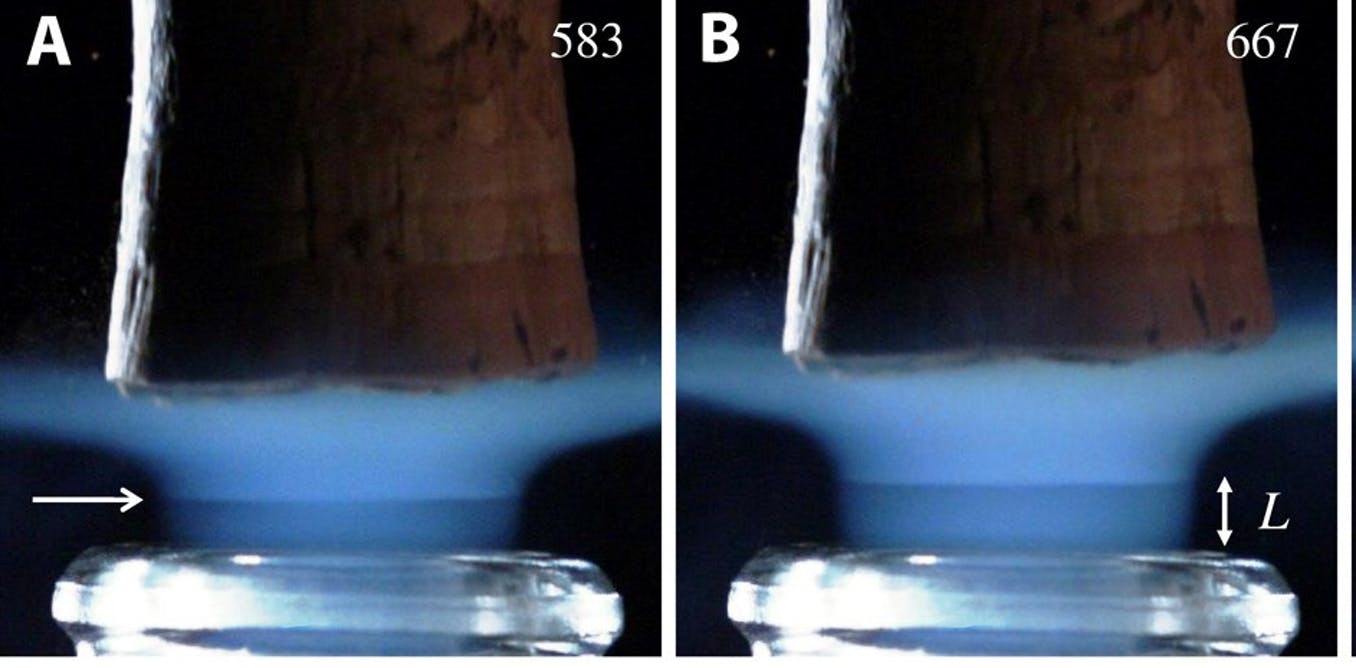
Did you notice the small horizontal line that intersects the blue column? About shock wave A feature of supersonic aircraft, known as Mach disks. It appears about 500 μs after unblocking, and progresses in the wake of the blockage before fading out after about 500 μs. Similar shock waves are found in the supersonic plume blown out of the reactors of a fighter jet or missile. During the first millisecond after the cork is ejected, the neck of the champagne bottle behaves somewhat like the nozzle of a rocket reactor. Who would have believed it!
Readers wishing to learn more about the world of champagne can refer to the book “A world of bubbles. Champagne or Fizzy Flag »Posted by Ellipses.

“Subtly charming problem solver. Extreme tv enthusiast. Web scholar. Evil beer expert. Music nerd. Food junkie.”