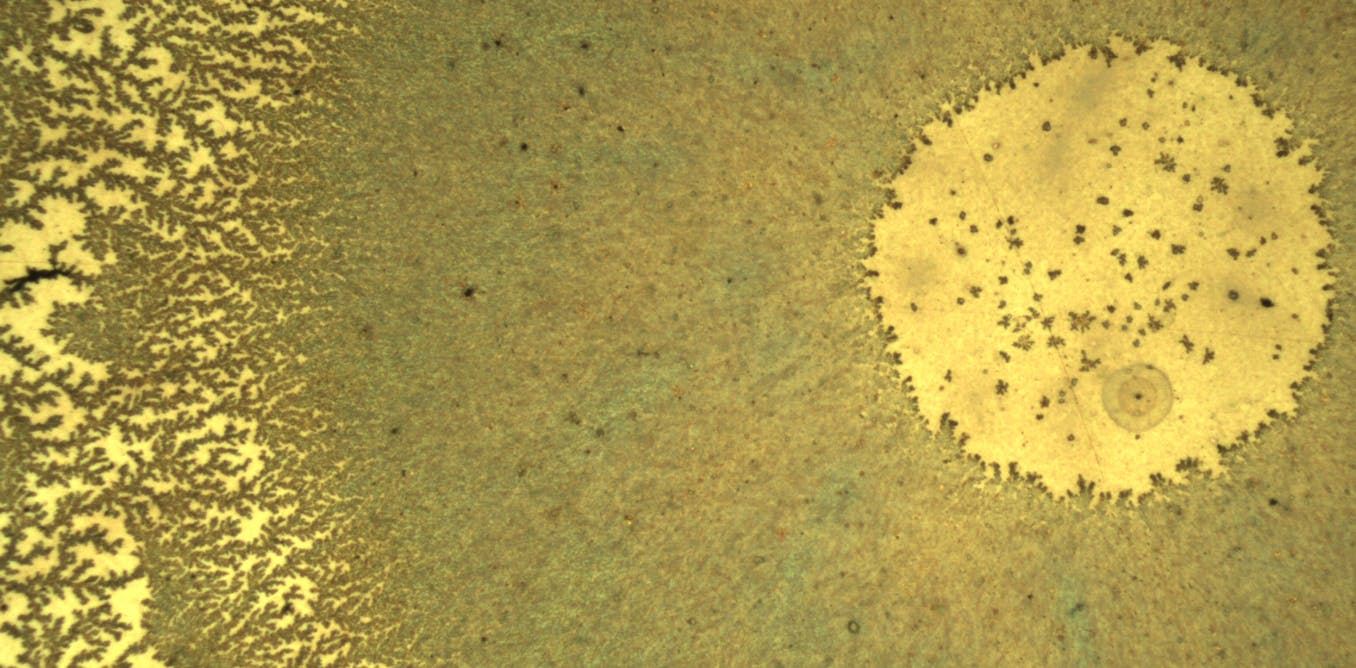
These beautiful bronze patterns appeared when a drop of water containing graphene oxide was placed under an optical microscope as the water evaporated.
By doing so, I help develop new applications based on graphene: batteries, “supercapacitors” (devices that allow high electrical energy to be charged and discharged very quickly compared to batteries) or even specific particle detectors.
To the left of the image are dark “tree” structures that resemble graphene oxide trunks and branches. The water did not evaporate homogeneously over the entire surface, this is referred to as “partial dehumidification”. This area is more outward than gout.
Then the drying progresses towards the center, the structure becomes dense and a dark area filled with graphene oxide is observed.
The graphene oxide agglomerates so much toward the edges of the drop that none remains in the center: at the end of drying, a tiny water droplet remains that contains very little graphene oxide. It’s the central circle visible in the image, and it’s mottled: only small pools of graphene oxide deposited on the surface in the final drying moments.
If we go back with trained eyes to the dark area between the dendrites and the central circle, we see that the color is heterogeneous. There are areas that tend towards green and others that turn towards brown … as well as some scattered black points through which the light does not pass.
These “synthetic colors”, also called “physical colors”, are created by various optical phenomena. This is for example thin film interference, like the ones that appear in an oil pool on wet ground. These colors show that this area does not have a constant thickness, and above all that it is not flat, but at least coarse.
Read more: Where do the wonderful colors of butterflies come from?
Why study the drip drying of graphene oxide?
Understanding the multiple inhomogeneities in a dry graphene oxide film is important for developing graphene-based applications by soft chemistry And at low cost.
Graphene conducts electricity very well, but it is not necessarily easy to work with. So we can use graphene oxide:graphene in sheets about ten nanometers to a few micrometers long, which can be dispersed in water thanks to the presence of hydrogen and oxygen atoms on the surface of the prisms. The disadvantage is that these atoms reduce the electrical conductivity by a factor of a thousand compared to pure graphene – it is necessary to carry out thermal decomposition, in an oven or, for example, with a laser, to partially restore it.
We seek to improve graphene/graphene-based electrodes in energy storage devices such as super capacitors.
For this, it is important to understand how droplets containing graphene oxide dry out. In fact, in a supercapacitor, the electrode is in contact with a liquid (or gel): the “electrolyte”. The quality of charging and discharging the capacitor depends on the quality of the interface between the electrode and the electrolyte (there should not be too much heterogeneity).
Supercapacitors can charge and discharge up to 1,000 times faster than conventional batteries such as Li-ion batteries with a life span (number of charge/discharge cycles) also 1,000 times longer. No loss in performance.
in the team Nanosciences and nanotechnology Research Department l’ECE ParisWe are studying these energy storage devices made up of electrodes Based on graphene oxide and ionic liquid electrolytes.
drying drops on substrates or “drop send”, is a simple, easy and rapid technique for preparing the surface and modifying its properties by depositing graphene oxide sheets on it from aqueous dispersions. Understanding the structures obtained during drying, as in the photo, as well as basic physical phenomena has become imperative with the development of printing technologies.Inks containing grapheneand in particular water-based (more “green”) inks containing graphene oxide.

“Subtly charming problem solver. Extreme tv enthusiast. Web scholar. Evil beer expert. Music nerd. Food junkie.”