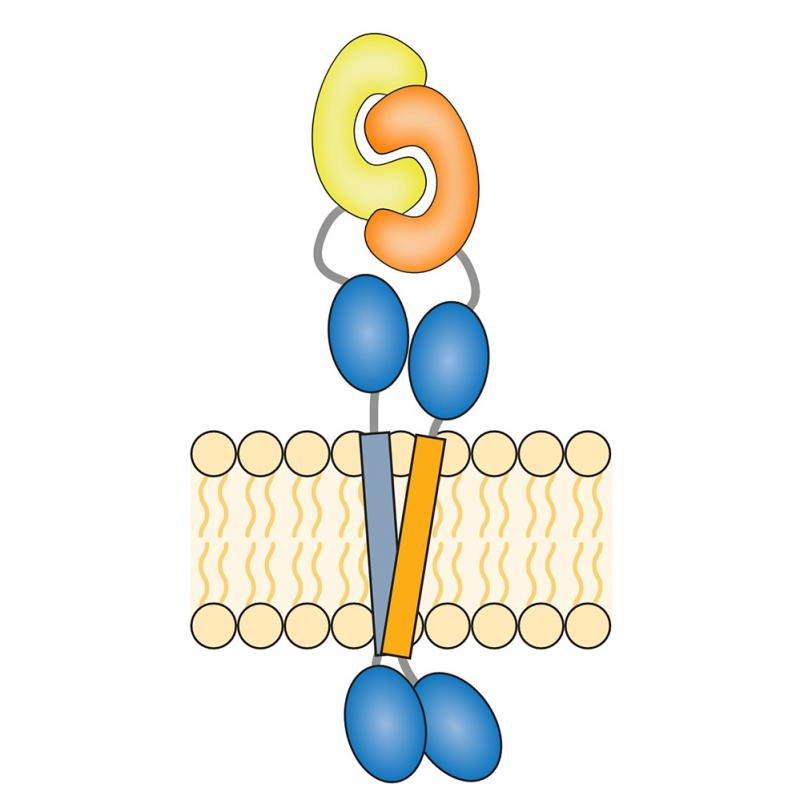
FLRT receptors act as ‘context identifiers’ that control the formation of different signaling complexes to regulate brain development. So far, research has focused on the extracellular domain of these receptors, which allows communication between two cells.
Thanks to an international collaboration involving French and British researchers, scientists have demonstrated a new type of FLRT2 receptor interaction. By combining molecular dynamics simulations with single-molecule fluorescence microscopy experiments, it was possible to quantify this interaction at the surface of the same cell. This interaction includes both the transmembrane domain of this receptor as well as the extracellular domain.
This work adds an additional level of complexity to all aspects of operating the FLRT receiver. It provides a new basis for understanding the structural mechanisms that determine the function of these receptors in tissue growth and suggests a new competitive binding mechanism between FLRT receptors on the same cell surface and those that can interact between two cells.

the shape : a model of the cis interaction of the FLRT2 receptor obtained by fusion
Molecular dynamics modeling (lower panel) quantifying transmembrane domain interaction via small-xxx-motifs and single-molecule fluorescence microscopy (right panel) allowing to follow the co-diffusion of these receptors).
To find out more :
The guidance and adhesion protein FLRT2 is dimerized in cis via the small-X . dimer3Small membrane patterns.
Jackson V, Hermann J., Tynan CJ, Rolf D. J., Corey Ra, Duncan L., Noriega M, Cho A, Kali AC, Jones EY, Sansom MSP, Martin Fernandez ML, Ciradaki E, Shavent M.
structure. 3 June 2022. doi: 10.1016 / j.str.2022.05.014.

“Subtly charming problem solver. Extreme tv enthusiast. Web scholar. Evil beer expert. Music nerd. Food junkie.”